Meta Analysis of Clinical Trials
Rachel Oughton
New medical treatments, for example drugs, operations, courses of therapy and many other interventions, are usually put through a series of rigorous trials before they are allowed to be used on the general population. These trials are usually randomized controlled trials, designed in such a way that the trial provides evidence of causality; that is, that the trial can infer with some confidence whether or not the intervention had an effect on the condition it is targetting. Controlled means that some participants are allocated to receive a control intervention (usually the standard current treatment) and randomized indicates that the method used to allocate participants to either the control group or the intervention group should be inherently random and unpredictable.
One of the first documented clinical trials was the investigation by James Lind into treatments for scurvy, aboard the HMS Salisbury in 1747.
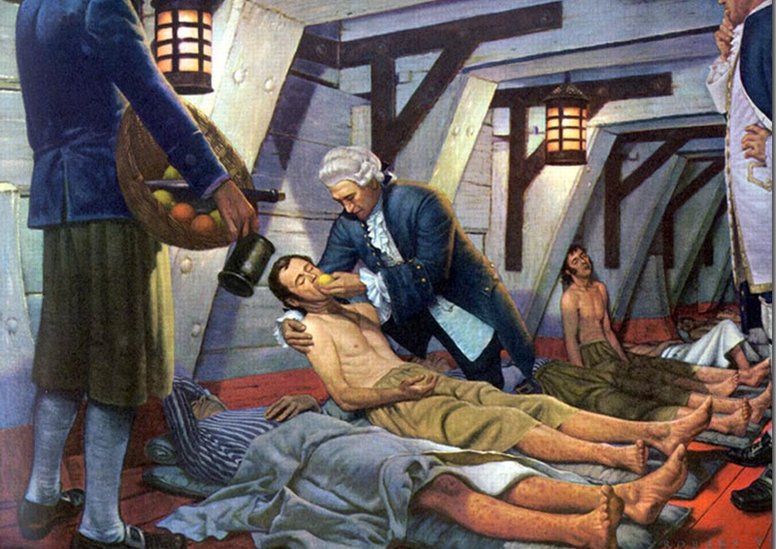
However since then, the field has progressed enormously in sophistication and rigour.
One aspect of the development of the field is that there are now many, many trials that are closely related to one another. They may test the same intervention but for different durations, or the same drug at different doses, or involve participants with slightly different eligibility criteria. A clinician who needs to decide whether or not to use a particular treatment may be faced with evidence from a large array of published trials that are all relevant to some greater or lesser extent. Meta-analyses attempt to combine results from from multiple trials, in order to present such clinicians with a digestible summary of the body of available evidence. One advantage of meta-analyses is that small, inconclusive studies may contribute to the overall picture.
There are many possible topics this project could cover, for example:
- The scrutiny of bias (eg. publication bias) in meta-analysis
- Using mixed effects models to estimate an overall effect
- Contrasting the Bayesian and Frequentist ways of approaching the meta-analysis of clinical trials
- Data collection - determining which trials are included in a particular meta-analysis
The statistical aspects of clinical trials are inextricably tied to the practical and clinical ones, and throughout a meta-analysis one must always be mindful of the ethical and practical implications and considerations. This grounding in the real world makes the subject challenging and rich, and this project would be hugely beneficial to someone considering a career in applied statistics (clinical or non-clinical).
Resources
Web
- The James Lind Library charts the history of the development of 'fair tests of treatments in healthcare'.
- This article disusses the place of meta analyses in the body of evidence generated by clinical trials
- This article gives some background about meta analysis, and some helpful R info (it's 10 years old now so some of the R advice is a bit outdated in terms of packages, but it's a useful intro)
- This article gives a very practical introduction to meta analysis in R
- The R statistical package can be downloaded from CRAN or RStudio and as the task view for clinical trials shows, there are many relevant packages.
Books
There are many books on clinical trials, most of which contain some information on meta-analysis, but some of the best are (you may have to go through the library website if these links require a login):- The meta analysis of clinical trials (Anne Whitehead)
- Introduction to Randomized Controlled Trials (CRC Press), John Matthews - a great overall introduction
- Bayesian approaches to clinical trials and health-care evaluation (Wiley), Spiegelhalter et.al. - a good introduction to the Bayesian approach. This paper (by the same authors) gives a good overview too.
Essential companion modules